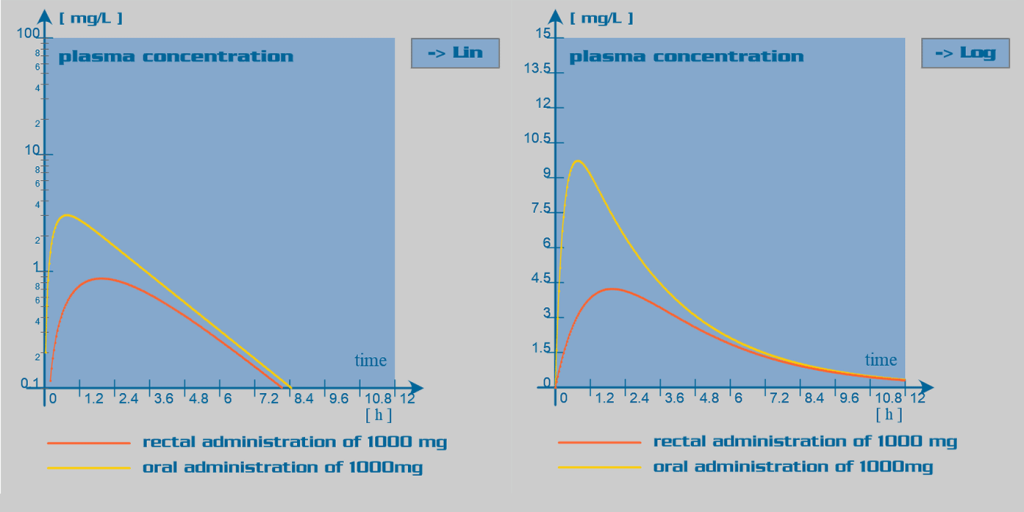
Paracetamol pharmacokinetic parameters
| Oral bioavailability (F) | 70%-90% |
| Rectal bioavailability (F) | 30%-70% |
| Clearance (CL) | 20 L/h |
| Volume of Distribution (Vd) | 65 L |
| Half-life (t1/2) | 2.5 h |
Description
Paracetamol (also called acetaminophen) is a widely used analgesic and antipyretic agent.
Paracetamol is well absorbed in the gastrointestinal tract. Oral bioavailability is dose dependant: with larger doses, the hepatic first pass effect is reduced due to overwhelming of the liver enzymatic capacity; and therefore, bioavailability is increased. Rectal administration of paracetamol is also feasible. In this case, bioavailability is inconsistent and in overall reduced, due to incomplete dissolution of the suppository in the rectum. The absorption rate through this route of administration is elongated.
Paracetamol is distributed throughout the body fluids in a homogeneous way. The analgesic activity is attributable to the small fraction that penetrates into the brain. Paracetamol given at therapeutic doses binds to plasma proteins at less than 20%. In case of intoxication, this proportion may increase to up to 50%.
Paracetamol is essentially metabolized in the liver by conjugation with glucuronic acid (55%) and sulfuric acid (35%). Hepatotoxic metabolites are produced in small amounts by the cytochrome P450 (isoenzyme CYP2E1). In the therapeutic plasma concentration range, this metabolite is detoxified by conjugation with glutathione. In case of intoxication the amount of this toxic metabolite increases and outweighs the amount of available glutathion, which can lead to hepatic failure and renal tubular necrosis.
Metabolites are excreted through the kidneys in the urine. Only 2-5% of the dose is excreted in an unchanged form in the urine. As a consequence of its short elimination half-life (1-3h), 24 hours after the ingestion of a single dose of paracetamol, 98% of the dose is eliminated.
Clinical implications
Since paracetamol is mostly eliminated by hepatic metabolism, patients with severe hepatic failure have a considerably longer elimination half-life, and therefore, paracetamol must be used cautiously in such patients.
Intoxication are not infrequent because paracetamol is one of the most widely used over-the-counter drugs. Paracetamol intoxication has important clinical implications as it can induce life-threatening hepatic and renal failure. This failure is probably due to the accumulation of toxic metabolites, caused by an increase in their production and a decrease in their detoxification due to depletion of glutathione stores. The treatment of intoxication is:
- gastric emptying and administration of active charcoal
- administration of N-acetylcysteine, which acts by replenishing the hepatic stores of glutathione (preferably by the oral route, otherwise intravenously)
- heamodialysis may be useful if started within 12 hours after ingestion of huge doses.