“Integral of the plasma drug concentration-time curve”
Description
The area under the plasma drug concentration-time curve (AUC) reflects the actual body exposure to drug after administration of a dose of the drug and is expressed in mg*h/L.
This area under the curve is dependant on the rate of elimination of the drug from the body and the dose administered. The total amount of drug eliminated by the body may be assessed by adding up or integrating the amounts eliminated in each time interval, from time zero (time of the administration of the drug) to infinite time. This total amount corresponds to the fraction of the dose administered that reaches the systemic circulation.
The AUC is directly proportional to the dose when the drug follows linear kinetics. The AUC is inversely proportional to the clearance of the drug. That is, the higher the clearance, the less time the drug spends in the systemic circulation and the faster the decline in the plasma drug concentration. Therefore, in such situations, the body exposure to the drug and the area under the concentration-time curve are smaller.
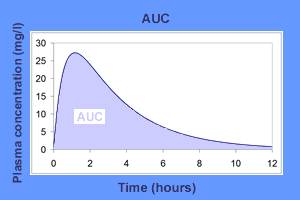
$$AUC = \color{cyan}{AUC0}-\color{cyan}2 + \color{pink}{AUC2}-\color{pink}4+\color{purple}{AUC4}-\color{purple}{6}+\\\color{yellow}{AUC6}-\color{yellow}{8}+\color{green}{AUC8}-\color{green}{10}+\color{HotPink}{AUC10}-\color{HotPink}{12}+\\\color{RoyalBlue}{AUC last}-\color{RoyalBlue}{infinity}$$
Clinical implications
During clinical trials, the patient’s plasma drug concentration-time profile can be drawn by measuring the plasma concentration at several time points. The AUC can then be estimated. Knowing the bioavailability and the dose, the clearance of the drug may be calculated by dividing the dose absorbed by the AUC. The clearance calculated is relatively independent on the shape of the concentration-time profile. This method gives precious information on the pharmacokinetic behavior of a drug on trial. It can also be used to study a change in the clearance of a drug in specific clinical conditions, such as disease or concomitant drug administration.
Related terms
Apparent clearance (CL’): In some pharmacokinetic trials, the bioavailability of the studied drug is not known. The apparent clearance, resulting from the dose divided by the AUC, reflects the drug’s clearance that does not take into account the bioavailability of the drug.
Assessment
$$AUC = {F * D \over CL}$$
After an iv bolus injection, the AUC can be calculated by the following equation:
$$AUC = { C(0) \over \lambda} $$
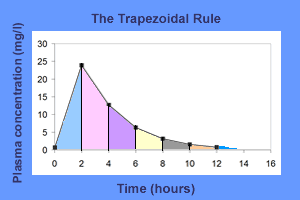
Trapezoidal rule: It consists in dividing the plasma concentration-time profile into several trapezoids and calculating the AUC by adding the area of these trapezoids.
AUC = Area under the concentration-time curve
F = bioavailability
D = dose
CL = clearance
C(0) = extrapolated plasma concentration at time 0
λ = elimination rate constant = CL/Vd