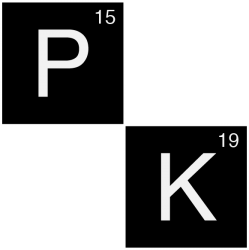
Rifampicin pharmacokinetic parameters
| Clearance (CL) | 10 L/h |
| Volume of Distribution (Vd) | 70 L |
| Half-life (t1/2) | 4 h |
Description
Rifampicin is used in the treatment of tuberculosis. It may also be used in the treatment of other infectious diseases such as in the eradication of meningococci from healthy carriers. Because of the ease with which many pathogens develop resistance to rifampicin monotherapy, it is often used in combination with other antibacterial drugs.
Rifampicin undergoes rapid and complete absorption after oral administration. Absorption is improved when the oral dose is taken on an empty stomach, as food may decrease the rate of absorption of rifampicin. Rifampicin absorption is very sensitive to changes in product formulation.
Rifampicin undergoes wide distribution into most body tissues and fluids, including the cerebrospinal fluid. It has a unique property of penetrating intracellularly. The protein binding is approximately 80%. Rifampicin crosses the placenta and may be found in maternal milk.
Rifampicin is extensively eliminated by intestinal and hepatic metabolism. The drug and its metabolites are largely excreted in bile and eliminated in stools. Rifampicin undergoes enterohepatic recirculation as does its metabolites. Only a small proportion of the dose is excreted unchanged in the urine (15-25%), giving the urine an orange color.
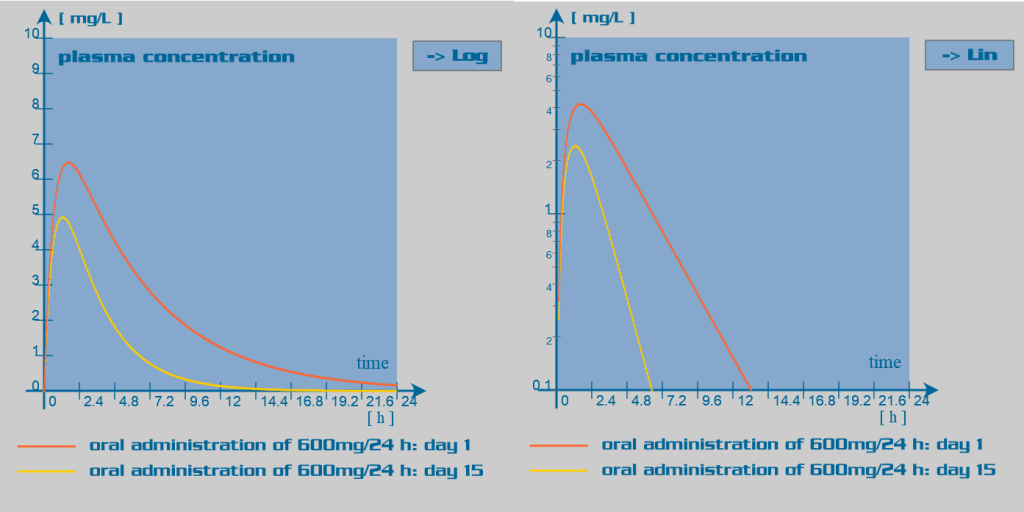
Since rifampicin is a potent enzyme inducer of the cytochrome P450 oxidase system, its administration is associated with numerous drug interactions. Rifampicin also has an inducer effect on its own hepatic metabolism. Therefore, repeated administration of rifampicin increases its oral clearance by inducing its own gut and hepatic metabolism or enhancing its biliary excretion. Rifampicin also induces the activity of the glucuronyltransferases, sulphotransferases and the efflux transporter P-glycoprotein.
Clinical implications
Because of the potential for drug interactions, patients receiving concomitant therapies which may be altered by rifampicin should be monitored closely to ensure efficacy of these therapies.
Use with caution and modify dosage regimen in patients with liver impairment, as metabolism can be significantly altered with marked reduced hepatic function, especially when used in combination with other hepatotoxins, (e.g. isoniazid) or inhibitors (e.g. antiretroviral drugs). No dose adjustments are needed for renal dysfunction or dialysis.