Theophylline pharmacokinetic parameters
| Oral bioavailability (F) | 96% |
| Clearance (CL) | 3 L/h |
| Volume of Distribution (Vd) | 35 L |
| Half-life (t1/2) | 8 h |
Description
Theophylline is used in the treatment of reversible airway obstruction due to chronic asthma, chronic bronchitis, or COPD. Both the bronchodilator and the adverse effects are closely related to drug concentration.
Theophylline is well absorbed when taken orally as a rapid-release tablet or when administered as a liquid solution. Bioavailability and rate of absorption of controlled-release preparations are more variable and influenced by formulation and food intake.
Theophylline distributes rapidly into fat-free tissues and body water. 40% is bound to plasma proteins, primarily to albumin, although blood pH values, the plasma protein content and the administration of concomitant drugs may vary this fraction. Theophylline passes freely across the placenta, into breast milk and into cerebrospinal fluid.
Theophylline is primarily eliminated by hepatic metabolism involving isoenzymes of the cytochrome P450 system. This metabolism has been assumed to follow first order kinetics (in some patients, nonlinear kinetics may be observed at therapeutic concentrations). Age and disease are the major endogenous factors influencing metabolism: clearance is markedly reduced in the neonates and elderly and increased in the first decade of life. Cirrhosis reduces clearance of theophylline, as does congestive heart failure. Exogenous factors such as concomitantly administered drugs, smoking and nutritional factors affect biotransformation by inducing or inhibiting the metabolizing enzymes: low-carbohydrate and high protein diet, active and passive smoking are associated with a significant increase in theophylline clearance.
Renal elimination of unchanged drug accounts for 10 to 15% of the total elimination of the dose in adults and may increase to up to 50% in neonates. Therefore, in a normal adult, renal disease has no significant effect on theophylline clearance.
Clinical implications
Estimation of the individual theophylline clearance should take into account the patients concomitant diseases, age and drug therapy. In patients with cor pulmonale, cardiac failure or end stage liver dysfunction, theophylline should be avoided if possible.
Because of intra- and inter-individual variability in the pharmacokinetics of theophylline, which may be increased by the presence of endogenous and/or exogenous factors, it is suitable to supervise theophylline therapy by therapeutic drug monitoring (TDM) for the target concentrations to be achieved.
Rifampicin is used in the treatment of tuberculosis. It may also be used in the treatment of other infectious diseases such as in the eradication of meningococci from healthy carriers. Because of the ease with which many pathogens develop resistance to rifampicin monotherapy, it is often used in combination with other antibacterial drugs.
Rifampicin undergoes rapid and complete absorption after oral administration. Absorption is improved when the oral dose is taken on an empty stomach, as food may decrease the rate of absorption of rifampicin. Rifampicin absorption is very sensitive to changes in product formulation.
Rifampicin undergoes wide distribution into most body tissues and fluids, including the cerebrospinal fluid. It has a unique property of penetrating intracellularly. The protein binding is approximately 80%. Rifampicin crosses the placenta and may be found in maternal milk.
Rifampicin is extensively eliminated by intestinal and hepatic metabolism. The drug and its metabolites are largely excreted in bile and eliminated in stools. Rifampicin undergoes enterohepatic recirculation as does its metabolites. Only a small proportion of the dose is excreted unchanged in the urine (15-25%), giving the urine an orange color.
Since rifampicin is a potent enzyme inducer of the cytochrome P450 oxidase system, its administration is associated with numerous drug interactions. Rifampicin also has an inducer effect on its own hepatic metabolism. Therefore, repeated administration of rifampicin increases its oral clearance by inducing its own gut and hepatic metabolism or enhancing its biliary excretion. Rifampicin also induces the activity of the glucuronyltransferases, sulphotransferases and the efflux transporter P-glycoprotein.
Clinical implications
Because of the potential for drug interactions, patients receiving concomitant therapies which may be altered by rifampicin should be monitored closely to ensure efficacy of these therapies.
Use with caution and modify dosage regimen in patients with liver impairment, as metabolism can be significantly altered with marked reduced hepatic function, especially when used in combination with other hepatotoxins, (e.g. isoniazid) or inhibitors (e.g. antiretroviral drugs). No dose adjustments are needed for renal dysfunction or dialysis.
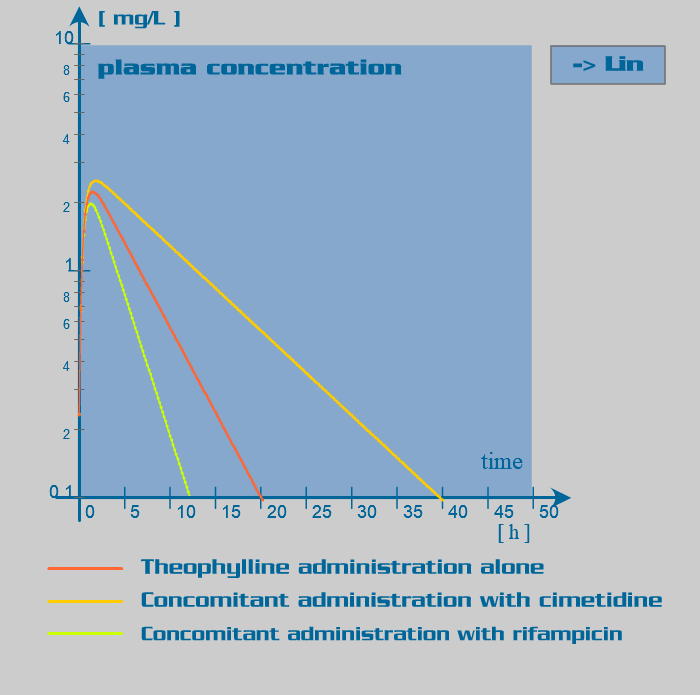
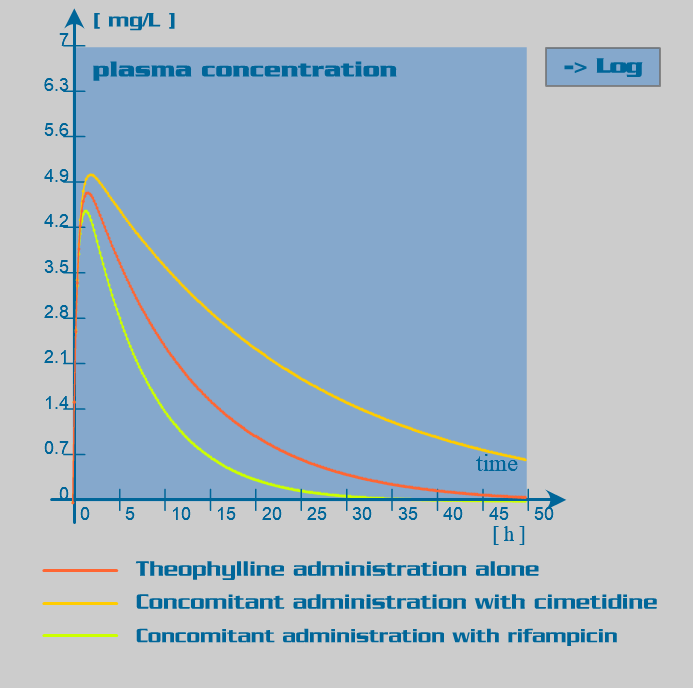
Theophylline pharmacokinetic parameters
| Induce metabolism significantly | Inhibit metabolism significantly |
| Phenobarbital | Cimetidine |
| Phenytoin | Ciprofloxacin |
| Rifampicin | Disulfuram |
| Hydrocarbons (smoke) | Oral contraceptives |
| Propanolol | |
| Erythromycin | |
| Fluvoxamine |